Have an upcoming abdominal surgery and are interested in seeing if you could potentially qualify for a clinical trial? Fill out the survey to see if you may qualify.
New advancements to potentially reduce surgical complications may be available to you

New advancements to potentially reduce surgical complications may be available to you
Have you been referred for gastrointestinal, gynecologic (such as a hysterectomy), urologic (such as prostate surgery), or another abdominal or pelvic surgery? You may want … to consider seeing if you qualify for a surgical clinical trial. Clinical trials are how new approaches to medicine are studied, and they afford participants the opportunity to play a role in potentially advancing surgical care. Clinical trials contribute to the development of new investigational treatment strategies, like developing tools to help doctors to better see — and avoid — areas of the body which are associated with post-surgical complications, such as the ureters. Filling out a quick survey is the first step to learning if participation might be right for you.
How Clinical
Trials Work
Thank you for your interest in learning more about surgical clinical trials! Every day, academic institutions, pharmaceutical organizations and private research facilities across the country are diligently working on making strides in scientific research in various clinical trials.
HERE’S A LOOK AT WHAT A SURGICAL CLINICAL TRIAL MIGHT INVOLVE:
WHO CAN PARTICIPATE?
Here are the qualifications interested potential participants need to meet in order to join a clinical research study for abdominal or pelvic surgery:
- Be at least 18 years of age
- Have a scheduled abdominal or pelvic surgery (e.g. hysterectomy, prostate surgery, bladder surgery, bowel surgery) in the next 1-3 months
- Have not undergone a kidney transplant
Only your surgeon can decide if you may be a suitable candidate for a surgical clinical trial. If you are interested in taking part in a study, your surgical team will provide additional information.
Clinical Trials Make A Difference
When you or a loved one participate in a clinical study, you provide valuable information that could eventually lead to a better treatment and — with the right insights — to better outcomes. Taking just a few minutes to learn more may help you better understand your care options, and play a part in helping to move surgical care forward. There is no obligation.
Why the Ureters Play Such a Key Role in Abdominal and Pelvic Surgeries
The ureters are narrow tubes that carry urine from the kidneys down to the bladder. Because they are thin, flexible, and located deep within the abdomen and pelvis, they can be difficult for surgeons to see clearly during procedures. In abdominopelvic surgeries—such as those involving the colon, uterus, or other pelvic organs—it is important for surgeons to identify exactly where the ureters run. Accidentally injuring or cutting a ureter can lead to complications that may require further treatment, such a sepsis, so being able to protect them is an important part of the surgical process.
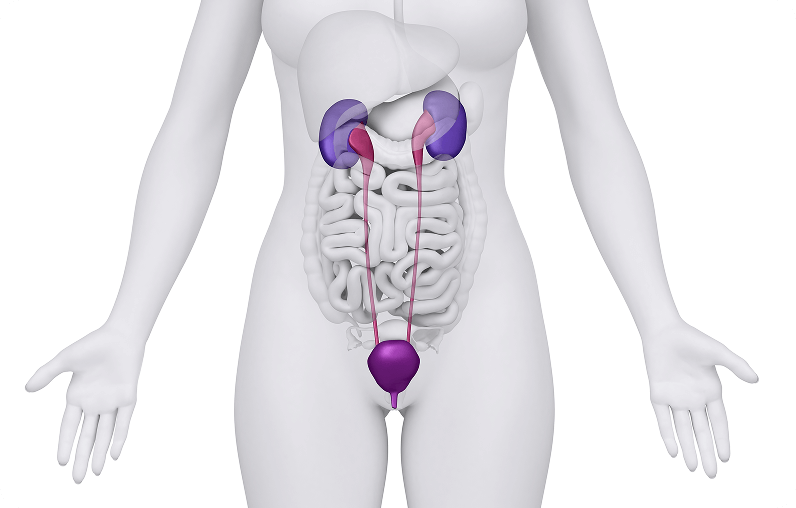
How Doctors Hope to Potentially Improve Surgical Outcomes
Doctors are continually looking for ways to make surgery safer and more precise. One area of research involves tools and investigational medicines that can help surgeons better visualize delicate structures like the ureters. The goal is to give surgeons a clearer “map” during procedures so they can correct the problem at hand while avoiding nearby tissues. With improved visibility, doctors hope to potentially be able to keep complication rates low and support smoother recoveries for patients.
≥ 55 percent of ureteral injuries are only discovered post-operatively when dramatic clinical signs emerge, such as stiffness, fever, abdominal pain, and/or bacteremia.1,2 Ureter injury also disproportionally impacts women.3,4
FREQUENTLY ASKED QUESTIONS
Who can join a surgical clinical research study?
Many surgical clinical studies are for individuals over the age of 18 who have been referred for or are scheduled to undergo an upcoming abdominal or pelvic surgery.
What is the purpose of a surgical clinical research study?
Clinical studies are intended to explore the potential benefits of an investigational medication, and in the case of an abdominopelvic surgical clinical trial, can be conducted in an effort to potentially offer surgeons better visibility of anatomical structures in an attempt to help potentially lessen injury and post-operative complications.
Are there any costs to participate in a surgical clinical research study?
There is no additional cost to participate in a clinical trial. Participants receive all study-related medical care and study-related medications at no cost. Likewise in many instances, patients may also enjoy benefits such as reimbursement for study-related travel.
Are there any risks to participating?
As all drugs and medical procedures carry a risk of side effects, the possibility that participants may experience some discomfort or other reactions as a result of an investigational treatment does exist. The potential risks will be explained before participants decide whether to participate, and your surgeon can help address any questions or concerns.
See If You Qualify
Still Have Questions?
Contact us anytime at help@clinicalenrollment.com.
- Source: Wu HH, Yang PY, Yeh GP, Chou PH, Hsu JC, Lin KC. The detection of ureteral injuries after hysterectomy. J Minim Invasive Gynecol. 2006;13(5):403–8.
- Sérénon V, Rouanet P, Charleux-Muller D, Eveno C, Poirot K, Trilling B, et al. Iatrogenic ureteral injury during colorectal surgery has a significant impact on patient outcomes: a French multicentric retrospective cohort study. Colorectal Dis. 2023 Jul;25(7):1433–45.
- Basic D, Ignjatovic I, Potic M. Iatrogenic ureteral trauma: A 16-year single tertiary centre experience. Srp Arh Celok Lek. 2015;143(3–4):162–8.
- Dowling RA, Corriere JN, Sandler CM. Iatrogenic Ureteral Injury. Journal of Urology. 1986 May;135(5):912–5.

